Introduction
In newly diagnosed diffuse large B cell lymphoma (DLBCL), the diagnosis-to-treatment (DTI) interval is associated with clinical outcomes: patients who initiated therapy early after DLBCL diagnosis have significantly worse event free survival (EFS) (Maurer JM et al. J Clin Oncol 2018;36:1603-1610). In relapsed or refractory (R/R) DLBCL, the relapse-to-treatment interval (RTI) has gained more relevance with the recent approvals of several novel therapies including chimeric antigen receptor (CAR) T-cell therapies, which require several weeks of manufacturing time, and bispecific antibody therapies that require several weeks of desensitization through dose ramp-up to achieve therapeutic levels. The association between RTI and patient outcomes in R/R DLBCL has not yet been reported.
Methods
We conducted an IRB-approved retrospective review of r/r DLBCL patients treated with rituximab, ifosfamide, carboplatin, etoposide (R-ICE) as second line treatment at three academic medical centers between 2010-2020. Characteristics at the time of relapse, including RTI and treatment outcomes were collected from the electronic medical record. Outcomes evaluated included disease response to R-ICE, subsequent autologous stem cell transplant (ASCT) and CAR T cell therapy, progression free survival (PFS) and OS. All analyses were done using R and its packages. The optimal cutoff values for RTI were determined using maximally selected rank test from MaxStat and survminer packages. The association between RTI and clinical outcomes was evaluated using Fisher test and Wilcoxon rank-sum test. Survival was calculated using the Kaplan Meier method, comparisons done using log-rank.
Results
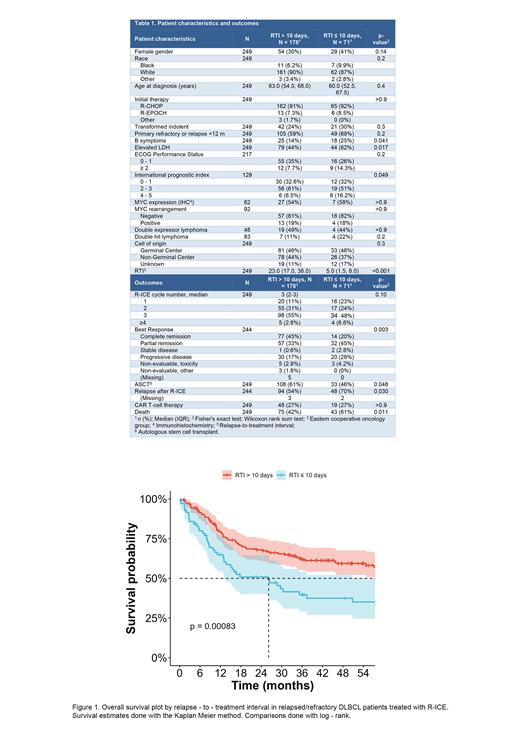
We identified 249 patients treated with second line R-ICE for r/r DLBCL with available RTI data. The median RTI was 18 days (IQR 9-31). The optimal RTI cutoffs calculated with maximally selected rank test were 7 days for PFS and 11 days for OS. An intermediate value of 10 days was chosen. At the time of relapse, age, gender, cell of origin and double expressor / double hit features were comparable among groups, but patients with an RTI of ≤ 10 days were more likely to have B symptoms, elevated lactate dehydrogenase, and intermediate or high IPI scores compared to those with longer RTI (table 1).
The RTI had a strong association with patient outcomes. Patients with RTI of 10 days or less had a lower rate of complete remission (20% vs. 45%, p = 0.003). After a median follow up of 53 months (IQR 36-73), median PFS for patients with RTI ≤ 10 days was 5.1 vs. 12.7 months for patients with RTI > 10 days (p <0.001). Four-year PFS estimates were 17.3% for patients with RTI ≤ 10 days vs. 35.3% for patients with RTI > 10 days. Median OS was 26 months for patients with RTI 10 days vs. 85 months for patients with RTI > 10 days (figure 1). Among patients with RTI of ≤ 10 days, 46% went on to receive ASCT, whereas 64% of patients with RTI > 10days had ASCT (p = 0.048).
On multivariate Cox proportional hazards analysis including RTI ≤10 days, age > 60 years at time of relapse, elevated LDH, and ECOG ≥ 2, the proportional hazards condition was met for both PFS and OS. Shorter RTI had a statistically significant association with PFS and OS, with hazard ratios (HR) of 1.80 (95% CI 1.27-2.56, p = 0.001) and 1.97 (95% CI 1.29-3.03, p = 0.002), respectively.
Conclusions
There is a strong association between RTI and the outcomes of R/R DLBCL patients treated with second line R-ICE chemotherapy. Our findings highlight the worse outcomes of patients with rapidly progressive, symptomatic R/R DLBCL and are particularly impactful in the era of CAR T-cells and novel immunotherapies, which can take many weeks to become available or effective. As with the DTI, clinical trials of DLBCL therapies should report RTI whenever possible. More effective and rapidly available treatment approaches should be designed to improve the outcomes of this high-risk patient population.
Disclosures
Caimi:SOBI: Honoraria; BMS: Consultancy; Genentech: Consultancy; Novartis: Consultancy; ADC Therapeutics: Consultancy; Lilly Oncology: Consultancy; Kite Pharma: Honoraria. Cashen:Kite Pharma: Consultancy. Hill:BeiGene: Consultancy; Bristol Myers Squibb: Consultancy; Genentech: Consultancy, Other: Advisory board, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Incyte: Consultancy; Gilead: Other: Advisory board; AstraZeneca: Consultancy; AbbVie: Consultancy, Other: Advisory board, Research Funding.